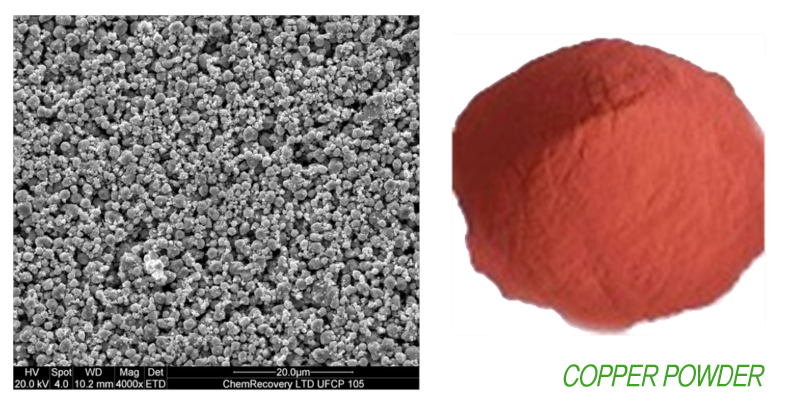
General Specification of Copper Powder
- Particle size 1 µm – 10 µm
- Purity as a copper metal 99.90 ± 0.1%
- Particle shape Spherical
- Electric & heat Conductive
- Colour reddish
- Apparent Density 2.8 – 2.9 gram/cm3
- Protected from Oxidation
Sample of analysis
ELEMENTWEIGHT %
Copper 99.90 ± 0.1
Aluminium 0.013
Silicon 0.031
Phosphorus 0.037
Sulphur 0.002
Chlorine 0.018
Calcium 0.012
Chromium 0.002
Iron 0.006
Nickel 0.009
Copper Powder is very valuable and has several different applications:
- Conductive paste in electronic devices
- Conductive inks
- Solar cells for solar energy devices
- Capacitor Chips
- Aerospace
- Chemical Catalysts
- Coatings
- Printing Inks
- Printed Circuits
- Injection Moulding
- Brazing/ soldering paste
- Sintered alloys/ products
- Antifouling paint for ships and boats
Material Safety Data Sheet
Copper, powder
Section 1 - Chemical Product and Company Identification
MSDS Name: Copper, powder
CAS No: 7440-50-8
Synonyms: None.
Company Identification:
Chemrecovery Industries limited
For information, call: +64 9 634 1960
NZ Emergency Number: 0800 243622
Other Emergencies: +64 9 6341690
Date of Preparation: August 2011
Section 2 - Hazards Identification
Classified as hazardous according to criteria in the Hazardous Substances (Minimum Degrees of Hazard) Regulations 2001.
Hazard classifications
6.1B (oral) Acutely toxic
6.1B (inhalation) Acutely toxic
6.4A Irritating to the eye
6.5B (contact) Contact sensitisers
6.6A Known or presumed human mutagens
6.9B (oral) Harmful to human target organs or systems
6.9B (inhalation) Harmful to human target organs or systems
9.1A (fish) Very ecotoxic in the aquatic environment
9.1A (crustacean) Very ecotoxic in the aquatic environment
9.1A (algal) Very ecotoxic in the aquatic environment
9.1A (other) Very ecotoxic in the aquatic environment
9.2D Slightly harmful in the soil environment
9.3A Very ecotoxic to terrestrial vertebrates
EMERGENCY OVERVIEW
Appearance: red to brown powder.
Warning! Obtain special instructions before use. Do not handle until all safety precautions have been read and understood. Flammable solid. Can be explosive when exposed to heat or flames. Causes respiratory tract irritation. Causes serious eye irritation. Causes skin irritation. May cause lung damage. Inhalation of fumes may cause metal-fume fever. May cause liver and kidney damage.
Target Organs: Kidneys, liver, lungs.
Potential Health Effects
Eye: Causes eye irritation.
Skin: Causes skin irritation. May cause skin discoloration.
Ingestion: Causes gastrointestinal irritation with nausea, vomiting and diarrhea. May cause liver and kidney damage. May cause genetic defects
Inhalation: Dust is irritating to the respiratory tract. Inhalation of fumes may cause metal fume fever, which is characterized by flu-like symptoms with metallic taste, fever, chills, cough, weakness, chest pain, muscle pain and increased white blood cell count.
Chronic: Prolonged or repeated skin contact may cause allergic skin reaction. May cause liver and kidney damage. May cause lung damage.
HSNO Labels
See Section 14 for Transport labelling
Section 3 - Composition, Information on Ingredients
CAS# Chemical Name Percent EINECS/ELINCS
7440-50-8 Copper 99-100 231-159-6
Section 4 - First Aid Measures
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin: Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Get medical aid if irritation develops or persists.
Ingestion: Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation: Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician: Individuals with Wilson's disease are more susceptible to chronic copper poisoning.
Section 5 - Fire Fighting Measures
General Information: As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Dust can be an explosion hazard when exposed to heat or flame. Flammable solid. May burn rapidly with flare burning effect. May re-ignite after fire is extinguished. Finely divided dusts may exhibit pyrophoric tendencies.
Extinguishing Media: Use dry sand, Met-L-X powder, or G-1 graphite powder. Contact professional fire-fighters immediately. Use dry sand, graphite powder, dry sodium chloride-based extinguishers. Dousing metallic fires with water may generate hydrogen gas, an extremely dangerous explosion hazard, particularly if fire is in a confined environment.
Flash Point: Not applicable.
Autoignition Temperature: Not applicable.
Explosion Limits, Lower:Not available.
Upper: Not available.
NFPA Rating: (estimated) Health: 2; Flammability: 2; Instability: 0
Section 6 - Accidental Release Measures
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks: Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up, then place into a suitable container for disposal. Scoop up with a nonsparking tool, then place into a suitable container for disposal. Avoid generating dusty conditions. Remove all sources of ignition.
Section 7 - Handling and Storage
Handling: Use personal protective equipment as required. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with skin and eyes. Empty containers retain product residue, (liquid and/or vapor), and can be dangerous. Keep away from heat, sparks and flame. Avoid ingestion and inhalation. Do not pressurize, cut, weld, braze, solder, drill, grind, or expose empty containers to heat, sparks or open flames.
Contaminated work clothing should not be allowed out of the workplace.
Storage: Keep away from sources of ignition. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances. Do not expose to air.
Section 8 - Exposure Controls, Personal Protection
Engineering Controls: Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate general or local explosion-proof ventilation to keep airborne levels to acceptable levels.
Exposure Limits
Chemical Name ACGIH NIOSH OSHA - Final PELs
Copper 0.2 mg/m3 TWA (fume); 1 mg/m3 TWA (dust and mist, as Cu) 1 mg/m3 TWA (dust and mist) 100 mg/m3 IDLH (dust, fume and mist) 0.1 mg/m3 TWA (fume); 1 mg/m3 TWA (dust and mist)
OSHA Vacated PELs: Copper: 0.1 mg/m3 TWA (fume, dusts, mists as Cu)
Personal Protective Equipment
Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin: Wear appropriate gloves to prevent skin exposure.
Clothing: Wear appropriate protective clothing to minimize contact with skin.
Contaminated work clothing should not be allowed out of the workplace.
Wash contaminated clothing before reuse
Respirators: Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - Physical and Chemical Properties
Physical State: Powder
Appearance: red to brown
Odor: none reported
pH: Not available.
Vapor Pressure: 1 mm Hg @1628C
Vapor Density: Not available.
Evaporation Rate:Not applicable.
Viscosity: Not applicable.
Boiling Point: 2595 deg C
Freezing/Melting Point:1083 deg C
Decomposition Temperature:Not available.
Solubility: Insoluble in water.
Specific Gravity/Density: 8.92
Molecular Formula:Cu
Molecular Weight:63.54
Section 10 - Stability and Reactivity
Chemical Stability: Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid: Ignition sources, dust generation, moisture, exposure to air, excess heat.
Incompatibilities with Other Materials: Strong oxidizing agents.
Hazardous Decomposition Products: Copper fumes.
Hazardous Polymerization: Has not been reported.
Section 11 - Toxicological Information
RTECS#:
CAS# 7440-50-8: GL5325000; GL7440000; GL7590000
LD50/LC50:
Not available.
Carcinogenicity:
CAS# 7440-50-8: Not listed by ACGIH, IARC, NTP, or CA Prop 65.
Epidemiology: No data available.
Teratogenicity: No data available.
Reproductive Effects: No data available.
Mutagenicity: Genotoxicity Effects. Several in vitro studies (Table 2-4) have examined genotoxic effects of copper in nonhuman systems. The results of the tests using prokaryotic organisms are equivocal. However, positive results have been observed in vitro (Table 2-4) and in vivo (Table 2-5) in mammalian systems. At low levels of copper (0.01-0.1 mM Cu as copper sulfate), DNA strand breaks were not observed in rat hepatocytes; however, strand breaks did occur at high concentrations (0.04 mM Cu as copper sulfate) (Sina et al. 1983). In vivo studies with Inbred Swiss mice showed that copper exposure resulted in chromosomal aberrations and micronuclei and sperm abnormalities (Bhunya and Pati 1987). Copper binds with the phosphate on nucleotides and nucleic acids of DNA. Its mutagenic potential may be the result of this binding (Sharma and Talukder 1987).
Although there is no data on the mutagenicity of copper in humans, in vivo studies and mammalian system in vitro studies suggest that copper is a potential human mutagen.
[ATSDR]
Neurotoxicity: No data available.
Other Studies: EndPoint:
Primary Organ: Renal toxicity (Kidney)
7.3.1 Oral
7.3.1.1 Copper(II) sulfate
The critical study is that of Hébert et al. (1993) which is described here. In comprehensive 90-day studies in both rats and mice (Hébert et al., 1993), in which copper(II) sulfate pentahydrate was administered in the feed at up to 8000 mg/kg in rats (up to 138 mg Cu/kg body weight per day) and up to 16 000 mg/kg in mice (up to around 1000 mg Cu/kg body weight per day), there were no overt signs of toxicity other than a dose-related reduction in growth (statistically significant in male and female rats from 67 and 138 mg Cu/kg body weight per day, respectively, and in male and female mice from 97 and 267 mg Cu/kg body weight per day). Microscopic examination of the tissues revealed hyperplasia and hyperkeratosis in the forestomach in both species (from 34 mg Cu/kg body weight per day in rats and from 187-267 mg Cu/kg body weight per day in mice), and liver and kidney effects in the rats only (from 67 mg Cu/kg body weight per day). In the rats, iron levels were reduced in the spleen, and haematological changes indicative of microcytic anaemia were observed at 34 mg Cu/kg body weight per day and higher. The NOEL was 17 mg Cu/kg body weight per day in rats, and 44 and 126 mg Cu/kg body weight per day in male and female mice, respectively. The liver and kidney effects observed in the rats in this study included inflammation of the liver and degeneration of the kidney tubule epithelium, and were similar to those found at higher doses (> 100 mg Cu/kg body weight per day) in more limited studies in rats (Haywood, 1980, 1985; Haywood & Loughran, 1985).
7.4 Long-term exposure chronic toxicity or carcinogenicity
The chronic toxicity/carcinogenicity of copper compounds has not been well characterized (see Table 11). Increased mortality and growth retardation or effects on the liver, kidneys or stomach have been observed in rats following long-term ingestion of 27-150 mg Cu/kg body weight per day as copper(II) sulfate, or 44-45 mg Cu/kg body weight per day as copper(II) acetate, in several limited studies. Long-term ingestion of copper(II) sulfate at 10 mg Cu/kg body weight per day induced marked hepatotoxicity in rabbits. An oral study in dogs did not show significant toxic effects at the highest dose of 8.4 mg Cu/kg per day, given as copper gluconate (Shanaman et al., 1972).
The available studies of the carcinogenicity of copper compounds in rats and mice have given no indication that copper salts are carcinogenic. However, the short duration or low level of exposure, the small group sizes employed, the limited extent of histopathological examination, or inadequate reporting limits the conclusions which can be drawn from such studies. The studies summarized in Table 11 are, therefore, inadequate to test the carcinogenic potential of copper compounds with any degree of certainty. In several studies, administration of copper compounds inhibited the development of tumours induced by known carcinogens (see Table 11).
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY. ENVIRONMENTAL HEALTH CRITERIA 200: COPPER. Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the World Health Organization, and produced within the framework of the Inter-Organization Programme for the Sound Management of Chemicals. World Health Organization Geneva, 1998 [INCHEM]
EndPoint:
Primary Organ:
Immunotoxicity
In an inhalation study in mice, single or repeated 3 h exposures to copper(II) sulfate aerosol resulted in significant immunosuppressive effects, including reduced bactericidal activity of the alveolar macrophages to Klebsiella pneumoniae and reduced resistance to infection by Streptococcus zooepidemicus. These effects were evident after a single exposure at 0.28 mg Cu/m3 and above and after 5 or 10 daily exposures at 0.06-0.07 mg Cu/m3. A NOEL was not established in these studies (Drummond et al., 1986).
INTERNATIONAL PROGRAMME ON CHEMICAL SAFETY. ENVIRONMENTAL HEALTH CRITERIA 200: COPPER. Published under the joint sponsorship of the United Nations
Environment Programme, the International Labour Organisation, and the World Health Organization, and produced within the framework of the Inter-Organization Programme for the Sound Management of Chemicals. World Health Organization Geneva, 1998 [INCHEM]
Remark: Inhalation:
Copper fumes cause irritation of the eyes, nose and throat, and a flu-like illness called "metal fume fever". Symptoms of metal fume fever include fever, muscle aches, nausea, chills, dry throat, cough and weakness. The symptoms disappear after 24 hours. Metal fume fever appears after exposure to about 0,1 mg/m3. Repeated or prolonged exposure to copper fumes may cause the skin and hair to change
colour. Renal damage reported. Nasal perforation reported in some cases. Chronic health affects, such as respiratory symptoms, gastrointestinal disturbances, nervous dysfunctions, dermatological and haematological changes and hepatomegaly. 75-100 copper workers, who were heavily exposed to copper dust of high purity, were examined over a 4-year period. The main findings were:
- in workers with Cu-S between 0,8-2,0 mg/l: increased alpha-2-globulins, redblood-cells and haemoglobin;
- in workers with Cu-S above 2,0 mg/l: different nervous symptoms, enlarged livers, abdominal distension and dermatological manifestations. Chronic irritative bronchitis was also observed.
Lung damage following chronic exposure to copper dusts and fumes in industry is not described.
The higher incidence of respiratory cancer reported in copper smelters is due to arsenic contained in the ore.
Source: Montanwerke Brixlegg Ges.m.b.H. Brixlegg
(69) Schaller, K.H., Triebig, G., Vanadium. in: Biological
iNDICATORS FOR THE Assessment of Human Exposure to
industrial Chemicals. Eds. Alessio, L., Berlin, A., Boni,
M., Roi, R. Comission of the european Communities, 1987 [IUCLID 2000]
Section 12 - Ecological Information
Avoid release to the environment.
Very toxic to aquatic life with long lasting effects.
Harmful to the soil environment.
Very toxic to terrestrial vertebrates.
Section 13 - Disposal Considerations
Chemical waste generators must determine whether a discarded chemical is classified as a hazardous waste. US EPA guidelines for the classification determination are listed in 40 CFR Parts 261.3. Additionally, waste generators must consult state and local hazardous waste regulations to ensure complete and accurate classification.
Section 14 - Transport Information
NZ Transport Code
Shipping Name: TOXIC SOLID, INORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: UN3288
Packing Group: II
Section 15 - Regulatory Information
New Zealand
HSNO Approval Code HSR002948
US FEDERAL
TSCA
CAS# 7440-50-8 is listed on the TSCA inventory.
Health & Safety Reporting List
None of the chemicals are on the Health & Safety Reporting List.
Chemical Test Rules
None of the chemicals in this product are under a Chemical Test Rule.
Section 12b
None of the chemicals are listed under TSCA Section 12b.
TSCA Significant New Use Rule
None of the chemicals in this material have a SNUR under TSCA.
CERCLA Hazardous Substances and corresponding RQs
CAS# 7440-50-8: 5000 lb final RQ (no reporting of releases of this hazardous substance is requir
SARA Section 302 Extremely Hazardous Substances
None of the chemicals in this product have a TPQ.
SARA Codes
CAS # 7440-50-8: immediate, delayed, fire.
Section 313
This material contains Copper (CAS# 7440-50-8, 100%),which is subject to the reporting requirements of Section 313 of SARA Title III and 40 CFR Part 373.
Clean Air Act:
This material does not contain any hazardous air pollutants.
This material does not contain any Class 1 Ozone depletors.
This material does not contain any Class 2 Ozone depletors.
Clean Water Act:
None of the chemicals in this product are listed as Hazardous Substances under the CWA. CAS# 7440-50-8 is listed as a Priority Pollutant under the Clean Water Act. CAS# 7440-50-8 is listed as a Toxic Pollutant under the Clean Water Act.
OSHA:
None of the chemicals in this product are considered highly hazardous by OSHA.
STATE
CAS# 7440-50-8 can be found on the following state right to know lists: California, New Jersey, Pennsylvania, Minnesota, Massachusetts.
California Prop 65
California No Significant Risk Level: None of the chemicals in this product are listed.
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols:
Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 7440-50-8: 0
Canada - DSL/NDSL
CAS# 7440-50-8 is listed on Canada's DSL List.
Canada - WHMIS
This product has a WHMIS classification of D2B, B4.
This product has been classified in accordance with the hazard criteria of the Controlled Products Regulations and the MSDS contains all of the information required by those regulations.
Canadian Ingredient Disclosure List
CAS# 7440-50-8 is listed on the Canadian Ingredient Disclosure List.
Section 16 - Additional Information
MSDS Creation Date: 22/08/2011
The information above is believed to be accurate and represents the best information currently available to us. However, we make no warranty of merchantability or any other warranty, express or implied, with respect to such information, and we assume no liability resulting from its use. Users should make their own investigations to determine the suitability of the information for their particular purposes. In no event shall Fisher be liable for any claims, losses, or damages of any third party or for lost profits or any special, indirect, incidental, consequential or exemplary damages, howsoever arising, even if Fisher has been advised of the possibility of such damages.